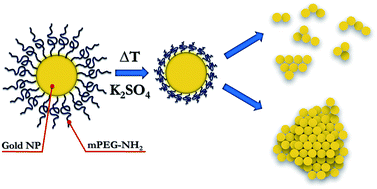
Surface modification of gold nanoparticles is very important in biological applications, not only to improve their stability, but also to functionalize their surfaces, making it easier to connect with biomolecules such as antibodies and enzymes. Commonly used thiol-containing ligands to modify gold nanoparticles, glutathione (GSH), mercaptopropionic acid (MPA), cysteine (cysteine), sarcosine (cystamine), dimercaptooctanoic acid (DHLA) and polyethylene glycol Alcohol (PEG), etc., of which the most common is mercapto polyethylene glycol (PEG-SH), which has the following advantages:
1.PEG is a high molecular polymer. When PEG is modified on gold nanoparticles, the stability of gold nanoparticles will change from static stability to steric stability, and the stable state is not easily affected by external factors;
2.PEG has no antigen and immunogenicity, non-toxicity and good biocompatibility;
3.PEG has no adsorption or other interaction with biomolecules in the physiological environment, which effectively reduces the adsorption of non-specific proteins and prolongs the cycle time;
4. Using PEG with different end groups, one section of which is connected to gold nanoparticles, and the active group at the other end can be modified to diversify the surface properties of gold nanoparticles.
The preparation method of PEGylated gold nanoparticles:
The main preparation methods of metal nanoparticles are physical method and chemical method.Among them, the physical methods mainly include vacuum evaporation method, soft landing method, electric dispersion method and laser ablation method;Chemical methods mainly include redox method, electrochemical method, seed method, microemulsion method, phase transfer method and template method.
Redox method:
In this method, a reducing agent is usually added to a solution containing high-valent gold ions, and the gold ions are reduced and aggregated into nanoparticles. The reducing agents used include classic trisodium citrate, sodium borohydride, phosphorus, cetylaniline, polyethylene glycol, polyaniline, and the like. Among them, the sodium citrate reduction method is the most classic, that is, using trisodium citrate as a reducing agent to reduce [AuCl4]- in a hot aqueous solution, the preparation method is simple, and the obtained particles are stable in nature.
Microemulsion method:
The method is to dissolve the surfactant in an organic solvent. When the concentration of the surfactant exceeds the critical micelle concentration (CMC), a liquid particle structure with hydrophilic polar heads inwards and hydrophobic organic chains outwards is formed. Solubilizes water molecules or hydrophilic substances. Microemulsions generally consist of four components, namely surfactant, co-surfactant (usually aliphatic alcohol), organic solvent (usually alkane or cycloalkane) and water. It is a thermodynamically stable system that can synthesize liquid particles of uniform size and particle size of 10-20nm.
Seed Growth Method:
The seed crystal growth method is to prepare gold nanoparticles through the seed crystal synthesis reaction. The advantage of this method is that the particle size distribution is narrower than that obtained by the one-step method, and gold nanoparticles with a particle size between 50-110 nm can be finally prepared by controlling the reaction conditions. ball. At the same time, the seed growth method has become the most popular method for the synthesis of colloidal gold nanorods due to its advantages of simple synthesis process, high yield, controllable aspect ratio and easy surface modification. The basic principle is that a certain amount of gold nanoparticle seeds is added to the reaction solution, and under the action of surfactant molecules, the seed particles grow directionally into gold nanorods with a certain aspect ratio.
Photochemical reduction method:
Photochemical reduction is the earliest method used to prepare gold nanorods. During the preparation process, gold acid is combined with rod-like cationic surfactant micelles to form ion pairs, and then electrons are transferred from metal ions to ligands under UV excitation. in the body, thereby reducing the gold ions to gold.
Surface PEGylation of gold nanoparticles:
The gold nanospheres were modified with thiol polyethylene glycol (PEG-SH). The modification process was as follows: 100μL of the above ligands at a concentration of 5X 10-4mol/L were added to 1ml of gold nanospheres. In the solution, stir it for 30S to mix well, and then put it in a 4°C refrigerator for later use.
Characterization of gold nanoparticles:
In order to fully and accurately characterize the properties of gold nanoparticles, multiple methods are usually used to analyze gold nanoparticles at the same time. The commonly used characterization methods are: ultraviolet-visible spectroscopy (Uv-vis), infrared spectroscopy (FI-IR), scanning electron microscopy ( SEM) or transmission electron microscopy (TEM), zeta potential or zeta potential (Zeta potential), X-ray diffraction (XRD), X-ray photoelectron spectroscopy, capillary electrophoresis (CE), etc.
Electron Microscopy (SEM, TEM):
SEM and TEM are intuitive methods to observe gold nanoparticles, which can clearly observe the particle morphology, size and dispersion.
Ultraviolet Visible (Uv-vis):
The nano-gold sol has a specific color in solution due to its plasmon resonance. The plasmon resonance absorption exhibited by the gold nanoparticles is different when the surface of the gold nanoparticles is different in size and shape. The plasmonic resonance of gold nanoparticles is divided into lateral stretching vibration and longitudinal stretching vibration. When the gold particles exist in the solution in a spherical shape, the lateral stretching vibration is mainly exhibited, and the Uv-vis absorption is around 520 nm. , linear, triangular flake or other non-spherical particles, the particles mainly exhibit longitudinal stretching vibration, the maximum absorption wavelength ranges from 600nm to 1400nm, the larger the maximum absorption wavelength, the greater the aspect ratio of rod and wire particles, The larger the edge-to-thickness ratio of the triangular piece, the solution showed different colors, such as gray, blue and dark brown.
Infrared Spectroscopy (FI-IR):
The molecules of each substance have a certain molecular structure and a certain energy level map, resulting in an infrared absorption spectrum, which brings the information of the molecular energy level map. For the molecules of the infrared spectrum, the interaction between the surface ligands and the gold nanoparticles can be understood, and the reaction mechanism and the mode of the effect of different modifiers on Au and the magnitude of the force can be analyzed simply and clearly.